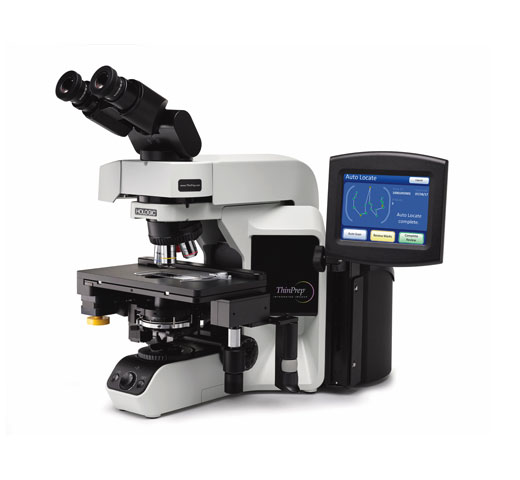
The Hologic ThinPrep® Integrated Imager is a device that uses computer imaging technology to assist in primary cervical cancer screening of ThinPrep® Pap Test slides for the presence of atypical cells, cervical neoplasia, including its precursor lesions (Low Grade Squamous Intraepithelial Lesions, High Grade Squamous Intraepithelial Lesions), and carcinoma as well as all other cytologic criteria as defined by the Bethesda System: Terminology for Reporting Results of Cervical Cytology
The ThinPrep Integrated Imager is an automated imaging and review system for use with ThinPrep Pap Test slides. It combines imaging technology to identify microscopic fields of diagnostic interest with automated stage movement of a microscope in order to locate these fields. In routine use, the ThinPrep Integrated Imager selects 22 fields of view for a cytotechnologist (CT) to review. Following review of these fields, the cytotechnologist will either complete the diagnosis if no abnormalities are identified or review the entire slide if any abnormalities are identified. The ThinPrep Integrated Imager also allows the physical marking of locations of interest for the cytopathologist.
The ThinPrep Integrated Imager is a combined system which uses computerized image analysis and automated microscope location to assist a cytotechnologist or pathologist to identify areas of a slide that are of most interest. Slides used with this system must first be prepared on the ThinPrep® 2000 System or ThinPrep® 5000 processors, and stained with ThinPrep® Stain. The ThinPrep Integrated Imager can be used as a conventional microscope when not used for ThinPrep® imaging. The ThinPrep Integrated Imager images the entire cell spot of the slide in approximately 90 seconds. The system acquires and processes image data from the slides to identify diagnostically relevant cells or cell groups based on an imaging algorithm that considers cellular features and nuclear darkness. During slide imaging, the alphanumeric slide accession identifier is recorded and the x and y coordinates of 22 fields of interest are stored in the system. After image processing, the device acts as an automated microscope, presenting the 22 fields containing the cells of interest to the cytotechnologist for review. The cytotechnologist uses the review control or touch screen to step through each of the fields of interest (Autolocate). Additionally, the review scope provides a method for automated marking of objects for further review. If the cytotechnologist identifies any of these fields as containing abnormal objects, that field may be marked electronically. The Integrated Imager will guide the cytotechnologist to conduct a review of the entire cell spot for any slide that has had fields electronically marked (Autoscan). The cytotechnologist determines specimen adequacy and the presence of infections during the review of the 22 fields of view presented by the ThinPrep Integrated Imager. Either of two methods can be used to determine specimen adequacy. The first method is to count cells and determine the average number of cells in the 22 fields of view presented by the Imager. The second method is to count and determine the average number of cells in 10 fields of view across the diameter of the cell spot. Either method will enable the cytotechnologist to determine if the minimum cells, as recommended by Bethesda System criteria, are present on the slide. At the conclusion of the slide review, electronically marked objects are manually marked on the slide by the cytotechnologist. Slide information is stored in the computer database including the x and y coordinates representing the electronically marked locations, and the status of the slide is designated as “complete”.

The cytotechnologist can review the slides immediately after each slide is imaged (sequential modality) or, as an alternative workflow for labs, slides can be imaged in succession, and coordinates stored in the computer database for later cytotechnologist or pathologist review (batched modality).
- Only personnel who have been appropriately trained should operate the ThinPrep Integrated Imager.
- All slides that undergo primary automated screening with the Integrated Imager require manual rescreening of the selected fields of view by a cytotechnologist or pathologist.
- The ThinPrep Integrated Imager is only indicated for use with the ThinPrep Pap Test. The ThinPrep Integrated Imager is only indicated for the ThinPrep Pap Test slides prepared with the ThinPrep® 2000 System and the ThinPrep® 5000 processor. The ThinPrep Integrated Imager is not indicated for the ThinPrep Pap Test slides prepared with the ThinPrep® 3000 processor.
- ThinPrep® slides with fiducial marks must be used.
- Slides must be stained using the ThinPrep Stain according to the applicable ThinPrep Integrated Imager slide staining protocol. Slides should be clean and free of debris before being placed on the system.
- The slide coverslip should be dry and located correctly.
- Slides that are broken or poorly coverslipped should not be used.
- Slides used with the ThinPrep Integrated Imager must contain properly formatted accession number identification information as described in the operator’s manual.
- Slides once successfully imaged on the Integrated Imager cannot be imaged again.
- The performance of the ThinPrep Integrated Imager using slides prepared from reprocessed sample vials has not been evaluated; therefore it is recommended that these slides be manually reviewed.